At TED2016, Jennifer Kahn talked about a bold new step in biotech: gene drives, the act of systematically removing a pesky gene from an entire species. Photo: Ryan Lash
To celebrate 10 years of TED Talks, we went back through the archives to show you how fast biotechnology has changed over the past ten years, and some interesting moments along the way.
We start with some history in a talk by James Watson, who co-discovered the first models of DNA’s structure with his partner Francis Crick, as a 23-year-old student at Cambridge in the early 1950s. Onstage in 2005, Watson talked about the breakthrough day when it all become clear. As he describes it, “Crick and I started building models, and I’d learned a little chemistry, but not enough. Well, we got the answer on the 28th February ’53, because of a rule which, to me, is a very good rule: Never be the brightest person in a room. And we weren’t. We weren’t the best chemists in the room.” Instead, their colleague, chemist Jerry Donohue, made an important correction to one of their earlier models for DNA’s structure that guided them to “the [DNA] base pairing — and Francis immediately said the chains run in absolute directions. And we knew we were right. It all happened in about two hours, from nothing to thing: If you just put A next to T and G next to C, you have a copying mechanism. So we saw how genetic information is carried.”
Following on this discovery, Watson and Crick asked further questions, like “what does this genetic information actually do?” In 1960, they came to understand more of the fundamental mechanisms that allowed DNA to transfer information, and discovered the three forms of RNA. These first discoveries paved the way for other scientists to explore life’s code.
The race to code the entire human genome started in the 1990s. The first human genome was sequenced (and published in Nature) by J. Craig Venter at Celera in 2001. The US-government-funded Human Genome Project completed its first human genome sequence two years later. The possibilities seemed endless, as Juan Enriquez told TED in 2003, imagining what impact we could expect this new tool to have across the economy, science and culture.
First, Enriquez mused, we might expect to see extinct species return to the planet. He gives this hypothetical example: “They take some cells out of an adult gaur‘s mouth, insert the code from that into a fertilized cow’s egg, reprogram the cow’s egg with a different gene code. When you do that, the cow gives birth to a gaur. We are now experimenting with bongos, pandas, elands, Sumatran tigers …”
Next, he predicted, the technology to sequence our gene codes would get faster and much cheaper, very rapidly: “It takes about $5 billion to sequence a human being the first time. Takes about $3 million the second time. We will have a $1,000 genome within the next five to eight years. That means each of you will contain on a CD your entire gene code.” This will introduce a new factor into our healthcare: our genetic data.
And finally, we’ll see the beginning of gene editing, and the birth of new industries for manufacturing vaccines and materials. “This changes all rules. This is life, but we’re reprogramming it.”
In 2005, J. Craig Venter talked about his sea-going expedition to map samples of the oceans’ DNA, mainly in microbes. “Less than 5,000 microbial species have been characterized as of two years ago, and so we decided to do something about it,” he said. On this trip, he discovered as many as 50,000 new species, adding entire chapters to the “book of life” on this planet.
“Microbes make up about a half of the Earth’s biomass, whereas all animals only make up about one one-thousandth of all the biomass,” he said. “If you ever swallow a mouthful of seawater, keep in mind that each milliliter has about a million bacteria and on the order of 10 million viruses.”
Next, Venter and his team started decoding the functions of different genes, recognizing which ones were necessary to survive in different environments — for instance, how microorganisms living at different depths adapted to more or less light.
As we learned more about how elegantly DNA stored our information, we started looking for bigger lessons on design and information storage. In 2008, Paul Rothemund told TED about how our long strands of DNA were able to fit into a compact space: by folding themselves into complex origami.
At this point, in 2008, it’s been seven years since the human genome was first sequenced, and Rothemund takes a minute to marvel at how the field known as biotechnology has grown and diversified, as we begin to grasp the magnitude of what we could learn from genetic code: “My friends, molecular programmers, and I … are interested in using DNA, RNA and protein, and building new languages for building things from the bottom up, using biomolecules, potentially having nothing to do with biology.”
In his own work, he took lessons from DNA’s shape that inform design for very small computers: “They took a DNA origami, organized some carbon nanotubes, made a little switch, wired it up, tested it and showed that it is indeed a switch. Now, this is just a single switch and you need half a billion for a computer, so we have a long way to go. But the origami can organize parts just one-tenth the size of those in a normal computer. So it’s very promising for making small computers.”
Meanwhile: “The 3.2 billion base pairs inside each of your cells is really a history of where you’ve been for the past billion years,” as Enriquez put it in his early talk. So at TEDGlobal in 2011, Svante Pååbo introduced us to some newly discovered clues in our DNA that linked us back to our Neanderthal ancestors.
As Pååbo says: “The two human DNA sequences go back to a common ancestor quite recently. Farther back, there is one shared with chimpanzees. And because these mutations happen approximately as a function of time, you can transform these differences to estimates of time, where the two humans, typically, will share a common ancestor about half a million years ago.”
By 2009, technology to sequence human genomes was so cheap and accessible that Ellen Jorgenson set up a DIY biohacking lab in Brooklyn, called Genspace.
“The idea,” she said, “is that if you open up the science and you allow diverse groups to participate, it could really stimulate innovation. Putting technology in the hands of the end user is usually a good idea because they’ve got the best idea of what their needs are. And here’s this really sophisticated technology coming down the road, all these associated social, moral, ethical questions, and we scientists are just lousy at explaining to the public just exactly what it is we’re doing in those labs. So wouldn’t it be nice if there was a place in your local neighborhood where you could go and learn about this stuff, do it hands-on? I thought so.”
Bonus: Jorgenson gave TED an update on how much it cost to process human genomes: “Reading and writing DNA code is getting easier and cheaper. By the end of this year, we’ll be able to sequence the three million bits of information in your genome in less than a day and for less than 1,000 euros.” Enriquez’s 2003 prediction on how rapidly this technology would develop had come true!
But as she suggests, as the technology became more complicated, it brought up hard ethical questions about human life and evolution. Should we edit ourselves? Our children? Other species?
Stewart Brand told TED in 2013 about some of the opportunities available to conservationists who were mourning extinct species.
“What if you could find out that, using the DNA in museum specimens, fossils maybe up to 200,000 years old could be used to bring species back, what would you do? Where would you start?”
“Well, you’d start by finding out if the biotech is really there. I started with my wife, Ryan Phelan, who ran a biotech business called DNA Direct, and through her, one of her colleagues, George Church, one of the leading genetic engineers, who turned out to be also obsessed with passenger pigeons and a lot of confidence that methodologies he was working on might actually do the deed.”
George Church was experimenting with a method of rebuilding ancient damaged genomes to bring back extinct species, Brand told us: “He has a machine called the Multiplex Automated Genome Engineering machine. It’s kind of like an evolution machine. You try combinations of genes that you write at the cell level and then in organs on a chip, and the ones that win, that you can then put into a living organism. It’ll work.”
After this talk, Chris Anderson said to Brand, “I suspect there are some people out there asking tormented questions: ‘Wait a minute, there’s something wrong with mankind interfering in nature in this way. There’s going to be unintended consequences. You’re going to uncork some sort of Pandora’s box of who-knows-what.’ Do they have a point?”
Brand responded, “Well, we interfered in a big way by making these animals go extinct.”
Over the next few years, we saw new tools built from our ability to read and process gene codes — and new ethical questions that were starting to keep us up at night.
In a challenging court case that became a TEDx Talk, Tania Simoncelli asked our audience if we should be able to patent a gene?
Simoncelli discussed a case she worked on that involved the BRCA1 and BRCA2 genes, which can be markers for breast cancer — and on which a biotech company had secured a patent in the 1990s.
What does that mean? It meant that you couldn’t give your gene to your doctors and ask them to look at it, without permission of the patent holder. It also meant that the patent holder had the right to stop anyone else from using that gene in research or clinical testing.
Luckily, there’s a long history of legal cases in the United States that ruled similar patents illegal. “Turns out that the Supreme Court has made clear that … you can’t patent products of nature — the air, the water, minerals, elements of the periodic table. And you can’t patent laws of nature — the law of gravity, E = mc2. These things are just too fundamental and must remain free to all and reserved exclusively to none.“
Present at court the day of the trial, she says, was “the co-discoverer of DNA himself, James Watson, who had submitted a brief to the court, where he referred to gene patenting as ‘lunacy.’” They won their case, protecting the public’s access to their own genetic code.
That same year, Jorge Soto demoed his tool for early cancer detection, leveraging a few new things we had learned about RNA and the recently discovered microRNAs, RNAs that influence gene expression.
“Unlike DNA, which is mainly fixed, microRNAs can vary depending on internal and environmental conditions, telling us which genes are actively expressed at that particular moment. And that is what makes microRNAs such a promising biomarker for cancer, because as you know, cancer is a disease of altered gene expression. It is the uncontrolled regulation of genes.”
“No two cancers are the same, but at the microRNA level, there are patterns. Several scientific studies have shown that abnormal microRNA expression levels creates a unique, specific pattern for each type of cancer, even at the early stages, reflecting the progression of the disease, and whether it’s responding to medication or in remission, making microRNAs a perfect, highly sensitive biomarker.”
With these tags, Soto’s team has been able to detect pancreatic, breast, lung and hepatic cancer with image detection in the cloud via a smartphone.
Meanwhile, a revolution in gene editing was brewing, with the advent of CRISPR, a basic, simple and reliable tool to edit genes quite precisely. In 2015 CRISPR’s co-inventor, Jennifer Doudna, walked us through the process — and laid out a vision for using it responsibly.
In 2015, Enriquez came back to TED with a bold talk on the ethical questions we’ll have when we start modifying the next human species. Which we definitely will. His talk lays out five basic principles for the bioethics around gene editing, things like: Take responsibility, and Accept diversity. And he closed by saying:
“This is the single most exciting adventure human beings have been on. It would be a crime for you not to participate in this stuff because you’re scared of it, because you’re hiding from it. You can participate in the ethics. You can participate in the politics. You can participate in the business. You can participate in just thinking about where we’re going to take the world. It would be a crime for all of us not to be aware.”
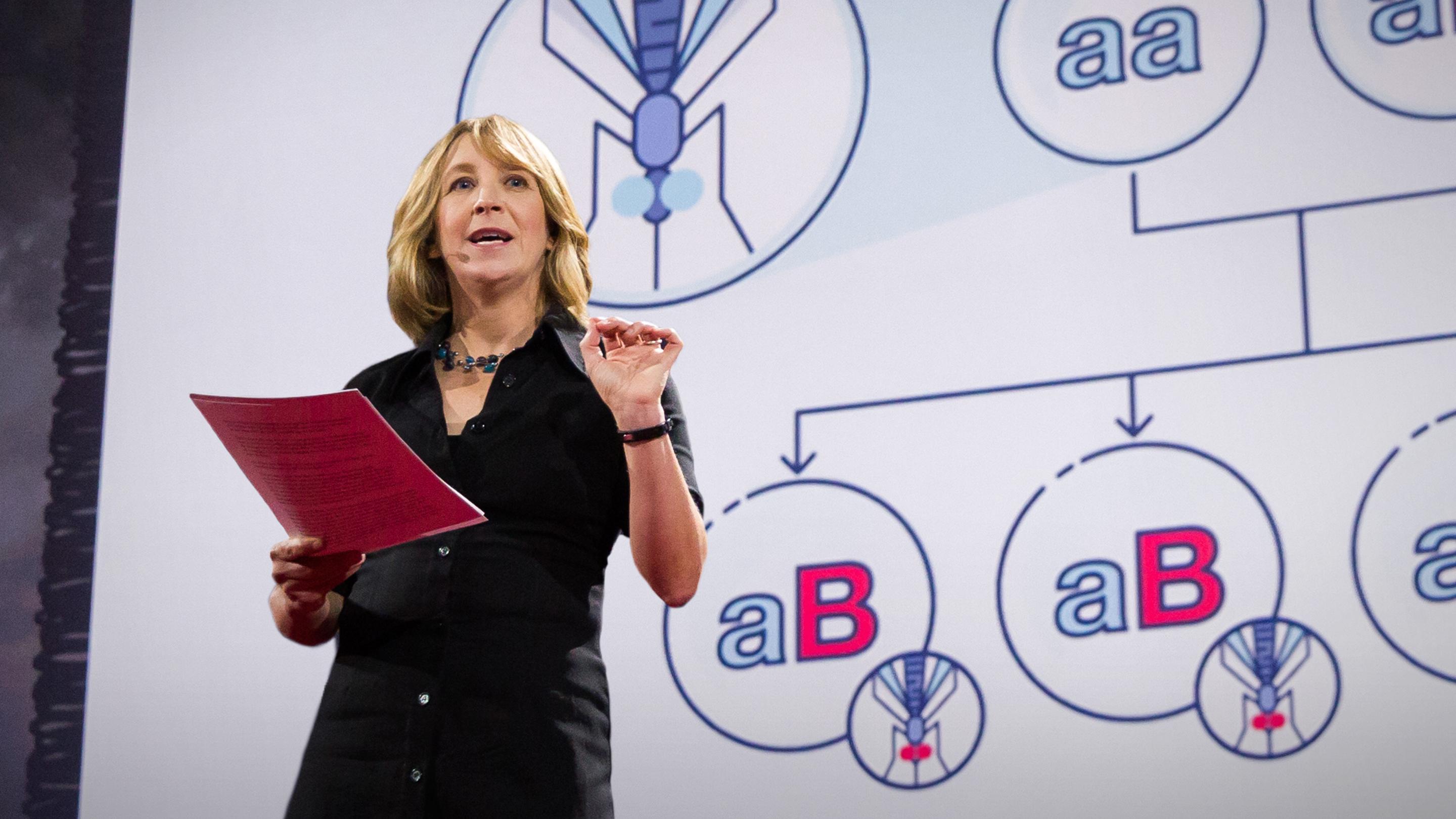
2016 brought new ethical questions. Jennifer Kahn challenged our audience to learn more about gene drives, global efforts to eradicate a disease through genetic engineering, empowered by CRISPR, which she calls “basically a word processor for genes. You can take an entire gene out, put one in, or even edit just a single letter within a gene. And you can do it in nearly any species.”
There are two sides to the genes drives story, she points out:
“The good news is that this opens the door to some remarkable things. If you put an anti-malarial gene drive in just 1 percent of Anopheles mosquitoes, the species that transmits malaria, researchers estimate that it would spread to the entire population in a year. So in a year, you could virtually eliminate malaria.”
“This is the bad news. Gene drives are so effective that even an accidental release could change an entire species, and often very quickly.”
It’s clear the first TED Talks online audience, back in 2006, was already getting hints of the world of today, 2016, a time in which we can zap genes one by one, create patient-specific medicines, and build computers that steal ideas from DNA folding. Our speakers have unpacked this quickly developing field for our audience with clarity, humor and insight. Now: what’s next?